 Acids & Bases
Acids & Bases pH Scale
Aim: I want to investigate how the universal indicator works and observe the changes.
Equipment: 500mL Beaker, Water, Sulfuric Acid ( Red Tip ), Magnetic Stirrer, Magnetic Stirrer Hotplate, Sodium Carbonate
( Green Tip ).
Results:
Discussion:
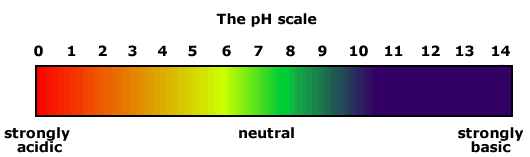
We first added in some sulfuric acid and the liquid went red, this is an indication of a strong acid with approximately has a pH of 1. Strong acids contain lots of hydrogen+ ions (H+). Then we added in some base ( Sodium Carbonate ), the base was soluble in water so it was an alkali. Alkalies release hydroxide ions (OH-) when dissolved in water. Where did the OH- come from? Na₂CO₃ this is sodium carbonate which will then react with water H₂O, they will dissociate or break up. The sodium carbonate has a positive charge and the carbonate has a -2 charge, in reaction with water it will accept an H leaving an OH-.
The liquid changed colour to a yellowy orange indicating a weaker acid which approximately has a pH of 5. The mixture has a few more hydrogen ions than the hydroxide ions. Next, we added in more base and it came to a green colour, this particular colour means it's neutral. There are equal amounts of hydrogen ions and hydroxide ions. Neutral has a pH of 7 on the scale. More base was added and then the mixture came to a dark blue colour, this is an indication of a weak base with approximately the pH of 10. It had a few more hydroxide ions than hydrogen ions. Lastly, we added in more sodium carbonate, to get a violet colour. This colour means it's a strong base with a pH of around 14. This mixture contains more hydroxide ions (OH-).
The liquid changed colour to a yellowy orange indicating a weaker acid which approximately has a pH of 5. The mixture has a few more hydrogen ions than the hydroxide ions. Next, we added in more base and it came to a green colour, this particular colour means it's neutral. There are equal amounts of hydrogen ions and hydroxide ions. Neutral has a pH of 7 on the scale. More base was added and then the mixture came to a dark blue colour, this is an indication of a weak base with approximately the pH of 10. It had a few more hydroxide ions than hydrogen ions. Lastly, we added in more sodium carbonate, to get a violet colour. This colour means it's a strong base with a pH of around 14. This mixture contains more hydroxide ions (OH-).
Example of Bases and Acids:
Strong Acid - Stomach Acid - pH of approximately 2
Weak Acid - Orange Juice - pH between 3 - 5
Neutral - Water - pH of 7
Weak Base - Baking Soda - pH between 8 - 9
Strong Base - Soapy Water - pH between 12 - 13
Evaluation:
During the experiment, there was one error, the magnetic stirrer did not stir the liquid. It would keep stirring and stopping. Later we realized the stirrer was too big for the magnetic hotplate however this was a simple issue and could be fixed. If you are to do this experiment I recommend using a stirrer which is smaller than the hotplate or simply stirring it yourself. Who invented the pH scale?
Facts:
- Acids taste sour and bases taste bitter.
- Acids and bases help neutralize each other.
- The pH of coke is 2.50. ( Acidic )



No comments:
Post a Comment
Note: only a member of this blog may post a comment.